A multidisciplinary research team has introduced a new photochemical strategy that enables precise control of drug activity deep within biological tissue using low-intensity near-infrared light. This advance was achieved through triplet-sensitized photoswitching of molecular compounds within the phototherapeutic window—where light penetration through biological tissue is maximized. This new approach opens a promising avenue for noninvasive and finely tunable photopharmacological modulation of physiological processes without the need for high-power illumination.
The ability to modulate biological processes with light has long been a central goal of photopharmacology, offering unmatched spatial and temporal precision. However, many existing approaches rely on ultraviolet or visible light, which penetrates tissue poorly and often requires high intensities that limit applicability in complex biological environments. Overcoming these constraints is essential for translating light-controlled molecular tools from laboratory settings into real-world biomedical applications.
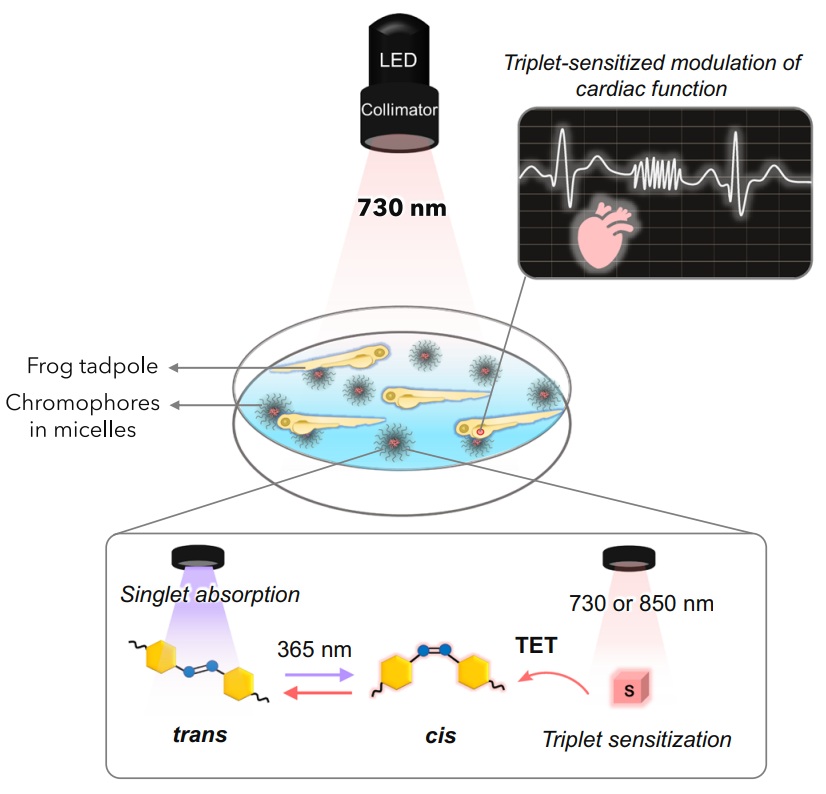
In a recent study published in Nature Communications, researchers introduce an innovative method based on triplet-sensitized photoswitching of azobenzene derivatives using red and near-infrared light. This strategy takes advantage of specially designed photosensitizers that absorb tissue-penetrating light and transfer energy to photoswitchable molecules, triggering structural changes that regulate biological activity. Importantly, this process operates at light intensities that are orders of magnitude lower than those required by conventional multiphoton or upconversion-based techniques. In other words, by applying synthetic and bio derived triplet-sensitizers, we can control a photopharmacologic drug by the use of near-infrared light, considerably enhancing the penetration depth of light into the living tissue.
PHOTOTHERAPORT researchers demonstrated the feasibility of this approach in vivo by combining a photosensitizer with an azobenzene-modified muscarinic receptor agonist to regulate the heatbeat in living frog tadpoles. When illuminated with continuous near-infrared light, the system enabled reversible modulation of heart rate without invasive procedures. This proof-of-concept illustrates that both the photosensitizer and the photoswitchable compound can function effectively within living tissue under physiologically compatible illumination conditions.
Beyond this specific demonstration, the study establishes a general framework for extending light-controlled molecular systems into deeper and less accessible biological regions. By reducing the need for high-energy light sources and leveraging wavelengths already compatible with clinical imaging and therapeutic technologies, triplet-sensitized photoswitching broadens the practical scope of photopharmacology.
This work marks an important advance in the development of noninvasive, light-responsive molecular tools. The ability to control biological functions using low-intensity near-infrared light opens new perspectives for precision modulation of physiological pathways, supporting future efforts to design adaptable and tissue-penetrant phototherapeutic strategies.
Reference article:
“Noninvasive cardiac modulation via triplet-sensitized photoswitching in the phototherapeutic window”. Lukas Naimovičius, Mila Miroshnichenko, Ekin Opar, Helen Hölzel, Masa-aki Morikawa, Nobuo Kimizuka, Manvydas Dapkevičius, Justas Lekavičius, Edvinas Radiunas, Karolis Kazlauskas, Víctor Cilleros-Mañé, Fabio Riefolo, Carlo Matera, Kevser Harmandar, Masahiko Taniguchi, Fabienne Dumoulin, Jonathan S. Lindsey, Pankaj Bharmoria, Pau Gorostiza & Kasper Moth-Poulsen. Nat Commun 16, 6377 (2025). https://doi.org/10.1038/s41467-025-61301-3