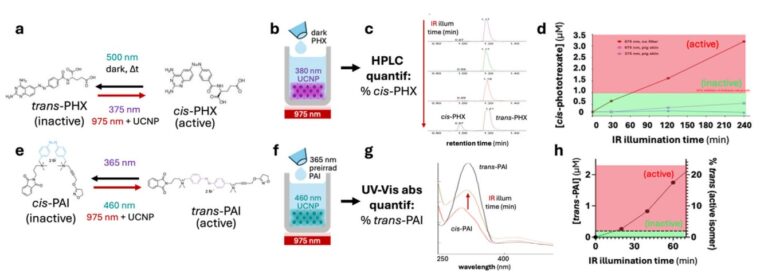
A recent scientific study led by PHOTOTHERAPORT project coordinator Pau Gorostiza, has uncovered a previously unrecognized mechanism in mitochondrial charge transport, revealing that protons and reactive oxygen species such as superoxide ions play a central role in mediating long-distance electron transfer between key proteins in the respiratory chain. This discovery enhances fundamental understanding of cellular energy production and may offer conceptual inspiration for future technologies.
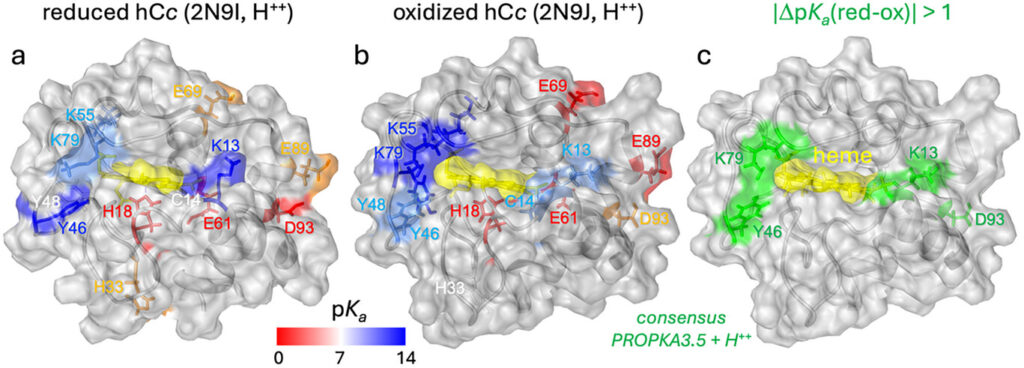
PHOTOTHERAPORT researchers at IBEC found that long-distance charge transfer between cytochrome c and respiratory complex III in mitochondria is facilitated not only by electrons but also through a coupled interaction with protons and superoxide ions — reactive oxygen species generated during normal cellular processes. The work, recently published in the journal Small, involved nanometric and single-protein experimental techniques to observe electron transfer phenomena that have been difficult to investigate using traditional methods.
Although this investigation is fundamental in nature, the underlying mechanisms it explores relate to core principles of charge and energy transfer at the nanoscale, which are also pertinent to the development of novel light-based therapeutic technologies such as those pursued by PHOTOTHERAPORT. By deepening our understanding of how charge carriers behave in complex biological environments, this discovery may provide valuable conceptual insights that inform the design of light-emitting devices, photoswitchable compounds, and other components under development in the project.
The findings indicate that charge transport efficiency is influenced by proton concentration, and that the presence of oxygen enhances the distance over which proteins can exchange charge. These results support a proton-coupled electron transfer (PCET) model in which the movement of electrons and protons is tightly linked, potentially involving dynamic networks of water molecules. The researchers also propose that superoxide anions contribute to the transport process, adding complexity to classical models of mitochondrial electron transfer.
From a biological perspective, optimizing charge transfer is essential for efficient cellular respiration, which underpins ATP production — the energy currency of life. While the direct medical or technological applications of these findings lie beyond the immediate scope of PHOTOTHERAPORT, the study expands the foundational knowledge of nanoscale charge dynamics, a field relevant to the project’s goals of integrating light-responsive systems with biomedical function.
By connecting advances in nanoscale bioelectric phenomena with applied biomedical engineering challenges, collaborations at the intersection of fundamental science and innovative therapy development continue to enrich the scientific landscape that PHOTOTHERAPORT seeks to transform.
Reference article:
“Long-Distance Charge Transport between Cytochrome c and Complex III is Mediated by Protons and Reactive Oxygen Species”. Anna Lagunas, Alexandre M. J. Gomila, Alba Nin-Hill, Alejandra Guerra-Castellano, Gonzalo Pérez-Mejías, Josep Samitier, Carme Rovira, Miguel A. De la Rosa, Irene Díaz-Moreno, Pau Gorostiza. Small (2025). DOI: 10.1002/smll.202501286