PHOTOTHERAPORT researchers met together in Barcelona last January to lay the first stone of this promising photobiomodulation project. The kick-off meeting was held at IBEC, the coordinator of the consortium.

PHOTOTHERAPORT focuses on the development of luminescent implants and light-activated drugs for innovative neuromodulation therapies. This forefront project is funded by European Innovation Council’s Pathfinder Open programme and will receive €3 million over 3 years for the preclinical study of these implants. The consortium comprises 8 institutions from 4 countries and is coordinated by the Institute for Bioengineering of Catalonia (IBEC) in Spain.
Focus on light-based therapies
Light-based therapies are a very promising tool to treat several health problems thanks to its ability to target specific regions of the body, in a very controlled way. However, despite their demonstrated therapeutic potential, these therapies encounter a common challenge: the attenuation of visible light through soft tissues and bones before it can reach the intended site, which increases with the depth of the target tissues and organs inside the body.
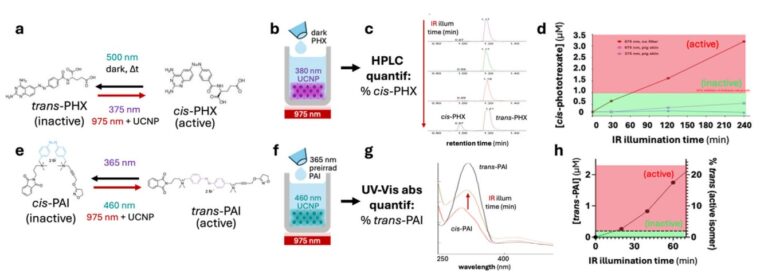
The innovative project PHOTOTHERAPORT proposes a solution to this challenge, with the design of implants, or PhotoTheraPorts, that can locally emit light when illuminated with an external light source. They incorporate upconversion nanoparticles that, when exposed to infrared light, emit higher-energy photons (visible or ultraviolet light). This allows the emission of light by the nanoparticles to be controlled remotely and non-invasively by externally applying infrared light, which does penetrate through the tissue and bone. Moreover, the shape and emission color of PhotoTheraPorts can be adjusted for various therapeutic purposes.
PhotoTheraPorts for treatment of pain, epilepsy and more
The research consortium will initially use this platform to directly photoinduce analgesia, an effect known as photobiomodulation, and compare it with clinically used lamps. The goal is to treat inflammatory pain more effectively in concealed regions of the spine.
The PhotoTheraPorts will subsequently be applied to photopharmacological neuromodulation therapies. For this purpose, photoswitchable drugs designed to synergize with the light emitter platform will be developed. These drugs activate only when exposed to a specific color of light. This second technology will undergo testing to mitigate neuropathic pain by illuminating specific regions of the spine.
Additionally, an effort will be made to inhibit the neuronal hyperactivity characteristic of focal epilepsy, which affects a limited region of the brain and is untreatable in 30% of epileptic patients.
Once the efficacy of PhotoTheraPorts for these pathologies has been proven, the platform has the potential to be applied to other conditions requiring treatments with localized action, such as arthritis, autoimmune diseases, infections, or transplants. The simplicity and versatility of this innovative platform aim to enhance pain management and patients’ quality of life, while also providing the healthcare system with ways to optimize medical costs and extend its benefits.
Project goal
Researchers aim to demonstrate, within 5 years, the safety and non-toxicity of the platform and to bring this new photobiomodulation therapy closer to clinical use, knowing that Photopharmacological development timelines are unpredictable and may be longer. If the project succeeds and achieves positive preclinical results for neuromodulation, it would be the first molecule of its kind to meet regulatory requirements.